Boyle's Law
Ever wondered why a balloon expands when you blow air into it or why a scuba diver's lungs compress as they dive deeper? The answer lies in Boyle's Law, a fundamental principle in physics that explains the relationship between the pressure and volume of a gas.
In this tutorial, we'll delve into the world of gases and explore how Boyle's Law works. We'll break down the complex concepts into easy-to-understand explanations, complete with real-world examples and practical applications. Whether you're a student, a curious mind, or simply looking to expand your knowledge, this guide will equip you with a solid understanding of Boyle's Law. Let's get started!
The volume of a fixed mass of gas is inversely proportional to its pressure at constant temperature.
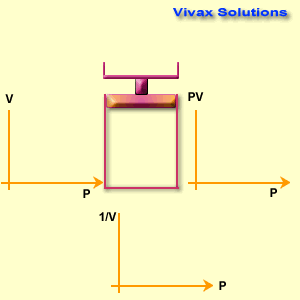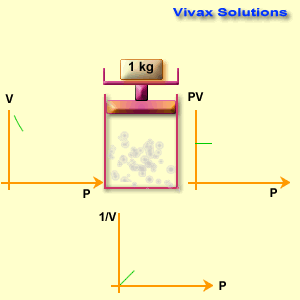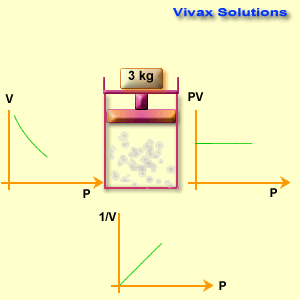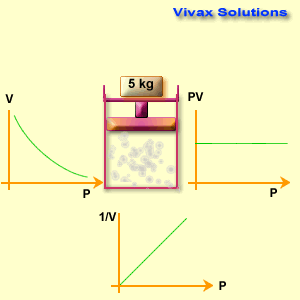
p ∝ 1 / v
p = k 1 /v
pv = k
Please move the slider and experiment with it.
P ∝ 1 / V
If the pressure and volume of a fixed mass of gas take the values of p1 v1and p2 v2 respectively,
p1 v1 = p2 v2
E.g.1
The pressure of a fixed mass of air is 20 Pa and volume is 8 cm3. Its pressure is doubled while keeping the temperature constant. Find the volume.
p1 = 20, v1 = 8, p2 = 40
According to Boyle's law,
p1v1 = p2v2
20 x 8 = v2 x 40
v2 = 4cm3
E.g.2
A scuba diver's tank contains 12 liters of air at a pressure of 200 atmospheres. What volume will this air occupy at the surface pressure of 1 atmosphere?
Using Boyle's Law, p1v1 = p2v2.
Plugging in the values, we get: 200 x 12 = 1 x V2.
Solving for V2, we find that the new volume is 2400 liters.
E.g.3
A syringe contains 5 mL of air at atmospheric pressure. The plunger is pushed in until the volume is reduced to 2 mL. What is the new pressure in the syringe?
Using Boyle's Law, p1v1 = p2v2.
Assuming atmospheric pressure is 1 atm, we get: 1 x 5 = P2 x 2.
Solving for P2, we find that the new pressure is 2.5 atmospheres.
E.g.4
A syringe contains 5 mL of air at atmospheric pressure. The plunger is pushed in until the volume is reduced to 2 mL. What is the new pressure in the syringe?
Using Boyle's Law, p1v1 = p2v2.
Assuming atmospheric pressure is 1 atm, we get: 1 * 5 = P2 x 2 .
Solving for P2, we find that the new pressure is 2.5 atmospheres.
E.g.5
A closed cylinder whose volume V, = 2.0 1 contains air at a pressure p, =
0.53 x 105Pa at 20°C. Then, the cylinder is submerged in water, hav�ing the same temperature and at a depth h = 1.2 m is opened. What volume of water enters the cylinder if the atmospheric pressure at this moment is 0.99 x
105Pa?
Using Boyle's Law, p1v1 = p2v2.
Assuming atmospheric pressure is 1 atm, we get: 1 * 5 = P2 x 2.
Solving for P2, we find that the new pressure is 2.5 atmospheres.
Using Boyle's law, p1V1, = p2V2 , where p2 is the pressure in
water at the depth h = 1.2 m, p2= 1.10 x 105Pa.
Therefore,
Vwater= V, — V2 = V, - p,V,/p2= V,(1 — p1 /p2)= 1.045 dm3.
The following animation shows how the lungs function, following Boyle's Law.
A Quiz on Boyle's Law
Please answer the following questions.
- An air column is trapped inside a capillary tube by a drop of mercury. The tube is placed
horizontally and the position of the mercury drop is noted. Then the tube is raised vertically with the open
end being at the top. What will happen to the mercury column? - go up or down.
- When a coke bottle is opened, air bubbles go up from the bottom. Explain this.
- A balloon filled with air is brought down in a lake; the deeper it goes, the more it tends to go up. Explain.
- A 1.5 litre can is filled with nitrogen at a pressure of 8 atmospheres. What
size can would be required to hold the same gas at a pressure of 4.0 atmospheres?.
- Can you apply Boyle's law when a car tire is being filled up?. Explain you answer.
- Two identical containers of volume 6cm3 contain the same gas at pressure 20 Pa and 12 Pa. They are linked by a
valve-tube. When the valve is opened, find the resultant
pressure of the system.
- An air column has been trapped in a tube sealed at one end by a mercury column, hav�ing a height, h = 19 cm. When the tube is placed with its open end downwards, the height of the air column is, 1, = 10 cm. If the tube is turned so that its open end is
at the top, the height of the air column is / 2 = 6 cm. Find the atmospheric pressure.